Covid-19 with RPP Genetic Testing is a comprehensive infectious disease test that detects the genetic material of SARS-CoV-2 (the virus causing Covid-19) along with a panel of other common respiratory pathogens, including viruses and bacteria. Using advanced molecular techniques like PCR, this test identifies multiple infections from a single sample, typically a nasal or throat swab. It is highly useful for diagnosing co-infections, distinguishing Covid-19 from flu, RSV, or other respiratory illnesses with similar symptoms. This combined testing approach enables timely and accurate treatment decisions, reduces transmission risk, and supports effective public health responses during respiratory infection outbreaks.
Choosing Covid-19 with RPP (Respiratory Pathogen Panel) genetic testing offers a comprehensive and efficient approach to diagnosing respiratory infections. Instead of testing for a single virus, this panel simultaneously detects Covid-19 along with multiple other respiratory pathogens, such as influenza, RSV, adenovirus, rhinovirus, and certain bacteria. This is especially valuable when symptoms overlap, ensuring accurate diagnosis and targeted treatment. The test uses highly sensitive molecular technology (PCR), providing rapid and reliable results. By identifying co-infections or the exact cause of illness, it helps reduce unnecessary treatments, hospitalizations, and the risk of disease transmission.



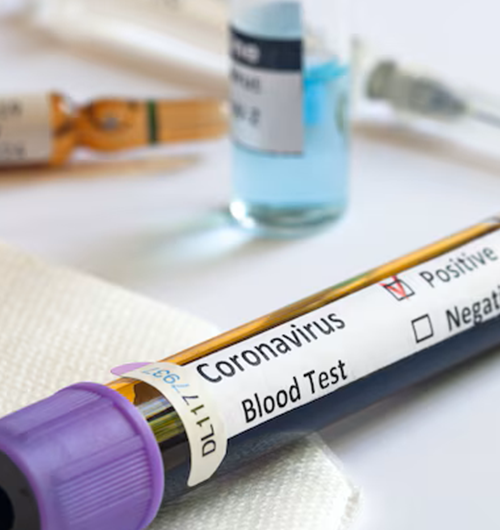

This testing is ideal for individuals experiencing respiratory symptoms where the exact cause is uncertain, especially during peak seasons of viral outbreaks. It is particularly important for high-risk groups, including the elderly, immunocompromised individuals, and those with underlying health conditions. Healthcare providers often recommend this test for hospitalized patients, those with severe symptoms, or in environments where rapid identification of infectious agents is crucial to prevent outbreaks. It’s also valuable before major medical procedures to rule out active infections.
You should consider this test if:

A healthcare provider collects a respiratory sample, typically using a nasopharyngeal swab, or sometimes a nasal or throat swab.
The sample is placed in a sterile tube with viral transport media and sent to a certified laboratory for analysis.
In the lab, viral RNA/DNA is extracted from the sample to isolate any genetic material from potential pathogens.
The extracted genetic material is analyzed using real-time polymerase chain reaction (RT-PCR) to detect multiple viruses and bacteria, including SARS-CoV-2.
Specialized equipment reads the PCR results to identify which pathogens are present in the sample.
A detailed diagnostic report is prepared and sent to the healthcare provider, usually within 24–48 hours, showing which pathogens were detected.
Based on the results, clinicians determine the appropriate treatment or isolation protocol to manage the patient’s condition effectively.

 01
01
Our fully integrated E-Lab platform offers online test ordering, real-time tracking, and secure digital reporting bringing convenience and control to both physicians and patients across the country.
 02
02
From routine blood tests to complex genetic panels, we leverage cutting-edge platforms like NGS, PCR, and AI-powered analytics for maximum accuracy and clinical relevance.
 03
03
With lab facilities based in Florida, we ensure faster processing times and direct access to our expert team. You get timely results backed by responsive, local customer support.
At E-lab, we are dedicated to innovation, collaboration, and seamless growth.
